 |
Figure 1. Electrochemical properties of P3-Na0.6[Li0.2Mn0.8]O2 layered oxides: (A) Typical charge-discharge curves at 0.1 C and 2.0 C rates; (B) At 0.1 C and 2.0 C rates Cycle performance.
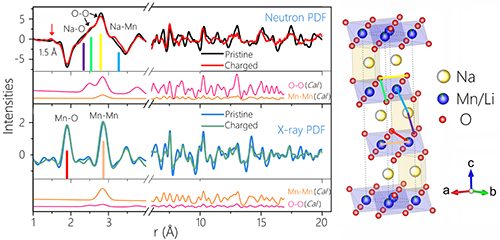
Figure 2. Comparison of nPDF and xPDF for initial and charged states of P3-Na0.6[Li0.2Mn0.8]O2
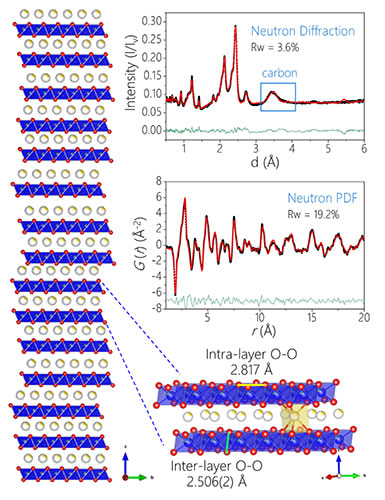
Figure 3. Analysis of charged state structure based on neutron diffraction refinement results
Embedded transition metal layered oxides (AMO2, A=Li+ or Na+, M=transition metal) are important lithium ion/sodium ion battery cathode materials. In traditional concepts, the redox reaction of the transition metal provides charge compensation during the ion-deintercalation material, so the capacity of the cathode material is limited by the redox ability of the transition metal in the layered oxide material. However, this traditional concept has been challenged with the discovery of lithium-ion battery-rich lithium layered oxide cathode materials (O3 structure Li[LixM1-x]O2). Lithium-rich materials have an ultra-high reversible specific capacity (300 mAh/g), but this source of capacity cannot be explained by only the transition metal redox. A large number of studies have shown that the lattice oxygen in the lithium-rich layered material participates in the process of gain and loss to provide additional capacity. In fact, not only lithium-rich materials, many layered oxide materials can realize the electrochemical process that oxygen participates in charge compensation to provide additional capacity. However, how lattice oxygen participates in charge compensation and how to achieve reversible oxygen valence has always been a hot topic in the debate. Explaining the relationship between crystal structure and the process of oxygen ion valence is the key to explaining its reaction mechanism.
Institute of Physics, Chinese Academy of Sciences/Beijing National Laboratory for Condensed Matter Physics (CPC) Key Laboratory of Clean Energy, Team E01, Ph.D. Rong Xiaohui, etc., Under the guidance of Associate Researcher Yan Xiqian and Researcher Hu Yongsheng, etc., with Oak Ridge National Laboratory, USA Liu Ye, Ph.D., Dr. Hu Enyuan and Dr. Yang Xiaoqing of Brookhaven National Laboratory, Yang Wanli, Research Fellow at Lawrence Berkeley National Laboratory, and Liu Yijin, researcher of the Stanford University Linear Accelerator Research Center, used advanced characterization methods such as neutron scattering and synchrotron radiation technology. A detailed study of the mechanism of sodium storage in a highly reversible P3-Na0.6[Li0.2Mn0.8]O2 sodium-ion battery anode material with oxygen ions involved in the redox reaction (Fig. 1) was conducted to clarify that the material achieves reversible oxygen The structural reason for the price change. The results of this study were published in Joule on the Structure-Induced Reversible Anionic Redox Activity in Na Layered Oxide Cathode.
Using a neutron pair distribution function (nPDF) combined with X-ray and neutron diffraction techniques, the research team studied the crystal structure of oxygen in the P3 positive oxide material before and after its participation in the electrochemical reaction, and related to oxygen. The short-range structural changes confirmed the reversible bulk structure of the material caused by the reversible oxidation of oxygen. This research method was used to study the oxygen charge-compensation-related crystal structure changes for the first time. The results are shown in Fig. 2. The neutron powder diffraction refinement results (Figure 3) show that the charged material structure is still a P-phase layered structure, but there is a large number of stacking faults. The oxygen occupancy obtained after the refinement is still 1, indicating that almost no lattice oxygen is lost after charging. By analyzing the above results, it is found that the crystal structure of the material plays a key role in controlling its reversible oxygen valence behavior: the P structure has a large interlayer spacing (relative to the O3 phase) and can tolerate the lattice distortion caused by the OO bond length change; At the same time, the larger interlayer spacing can effectively inhibit the migration of cations to the alkali metal layer during the charging process (phase change of the layered spinel structure occurring in the lithium-rich material), and maintain a stable layered structure, thereby making the oxidation and reduction of oxygen ions. The reaction is reversible.
The study clarified the mechanism of the reversible oxidation-reduction reaction of oxygen ions from the perspective of material structure, and provided new ideas for designing high-voltage, high-capacity lithium/sodium-ion positive electrode materials with stable reversible oxygenation behavior; The study introduced a neutron-to-distribution function (nPDF) as a powerful tool and broadened the research dimension.
The research work was supported by the National Key Basic Research and Development Program (973 Program), the National Outstanding Young Scientists Fund, the National Natural Science Foundation Innovation Research Group, and the 100-person plan of the Chinese Academy of Sciences.
The brick effect
tiles are the perfect choice for enhancing home interiors with
applications such as brick siding, brick fireplaces, brick
effect tiles kitchen backsplash.
Thin Brick Tile can replace ceramic tiles, vinyl siding, wood or aluminum siding. Exposed Brick Effect Tiles can be easily installed on
almost any interior wall without increasing the weight and cost of the concrete
foundation.
How to install thin brick?
The
construction wall must be clean, level and reasonable in structure to receive
adhesives and bricks.
Choose from a variety of color combinations, arrange the best results and then install the wall.
When installing, first put the corners, then stick the plane, after the adhesive is full, press the adhesive to squeeze around the cultural bricks to avoid the empty bricks in the installation.
Depending on the construction needs, you can easily cut brick veneer tile with a tile saw or an angle grinder.
Seam treatment, fill the bricks, remove the excess mortar, and try to avoid the mortar staining the brick surface.
Brick Effect Tiles,Brick Effect Tiles Kitchen,Exposed Brick Effect Tiles,Real Brick Effect Tiles
UMS New Materials Industry Co.,LTD , https://www.ums-factory.com